Overview of Testing Solutions
There are three types of lab testing methods/platforms available. They detect the virus or the patient’s response to the virus in different ways:
- Molecular
- Viral Antigen
- Serology (antibody) Tests
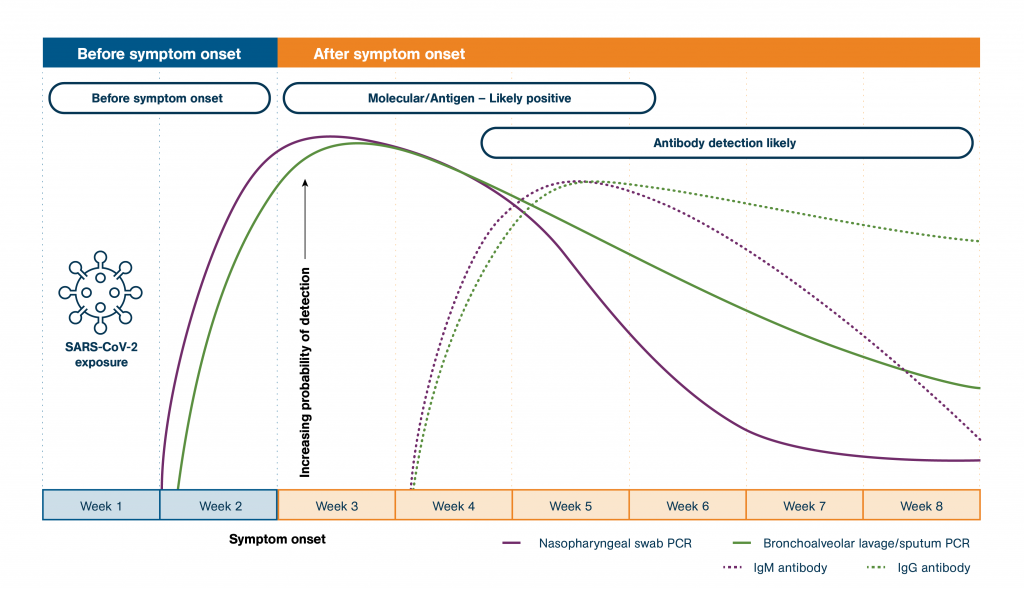
During the rise of viral RNA and viral antigen levels, a molecular (PCR or isothermal nucleic acid amplification based) or viral antigen test detects the presence of the virus. Often times, these molecular and viral antigen tests can be used as the primary basis for diagnosing your patient’s condition. Rather than going through the normal approval process, the U.S. Food and Drug Administration (FDA) is permitting all COVID-19 molecular and viral antigen tests to be cleared through its emergency use authorization (EUA) process during the pandemic. COVID-19 tests that go through the EUA process can request and secure a CLIA categorization.
Serology antibody tests detect the IgM and IgG antibodies that indicate your patient has developed an immune response to the virus but does not provide definitive evidence of a current infection. While experts say these tests will be important in the future, we have not seen widespread guidance on how these tests should be used. Serology antibody tests continue to be a focus in healthcare with the U.S. Food and Drug Administration (FDA) releasing very specific updates on these tests. The FDA has issued emergency use authorization (EUA) for some serology antibody tests. However, some serology antibody tests are being marketed without EUA. COVID-19 antibody serology tests without EUA currently default to a high complexity status. In addition, the accuracy and quality of those tests may vary greatly.
To know more about Serology Antibody tests, you may visit FDA.gov and review the FDA’s Revised Policy on Antibody Tests and the FDA’s EUA Authorized Serology Test Performance pages.